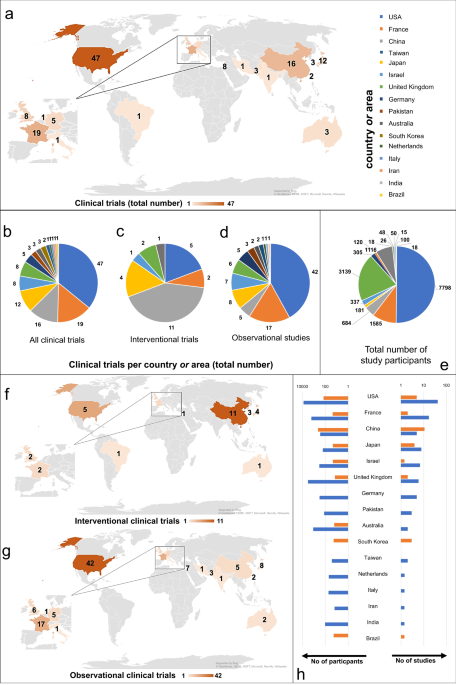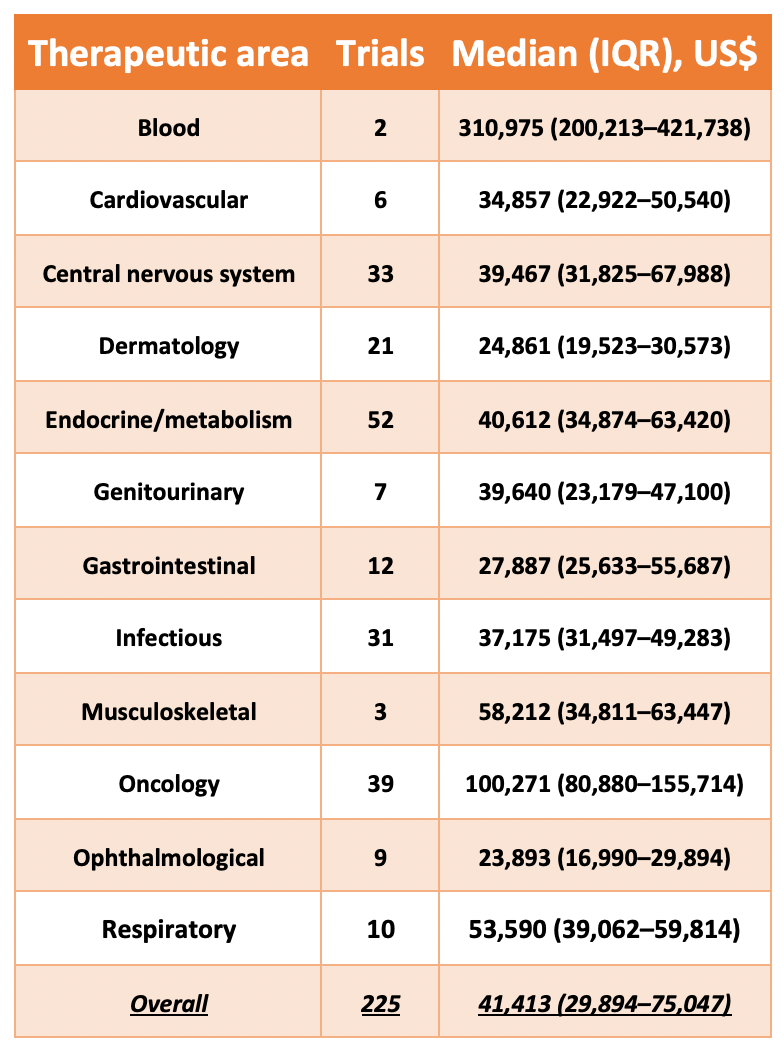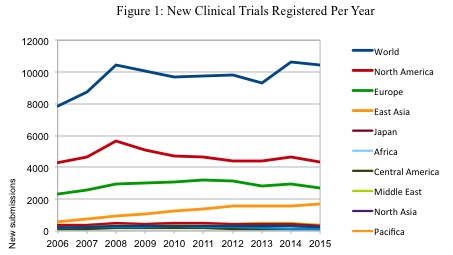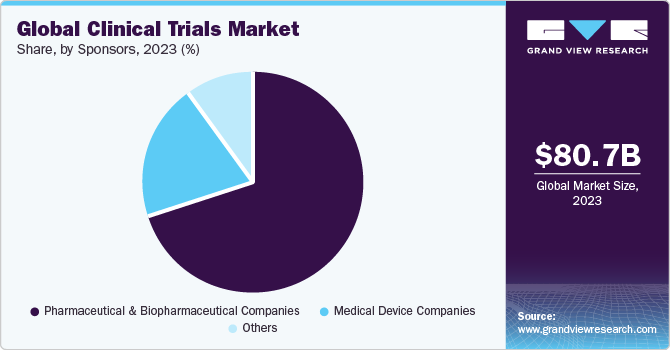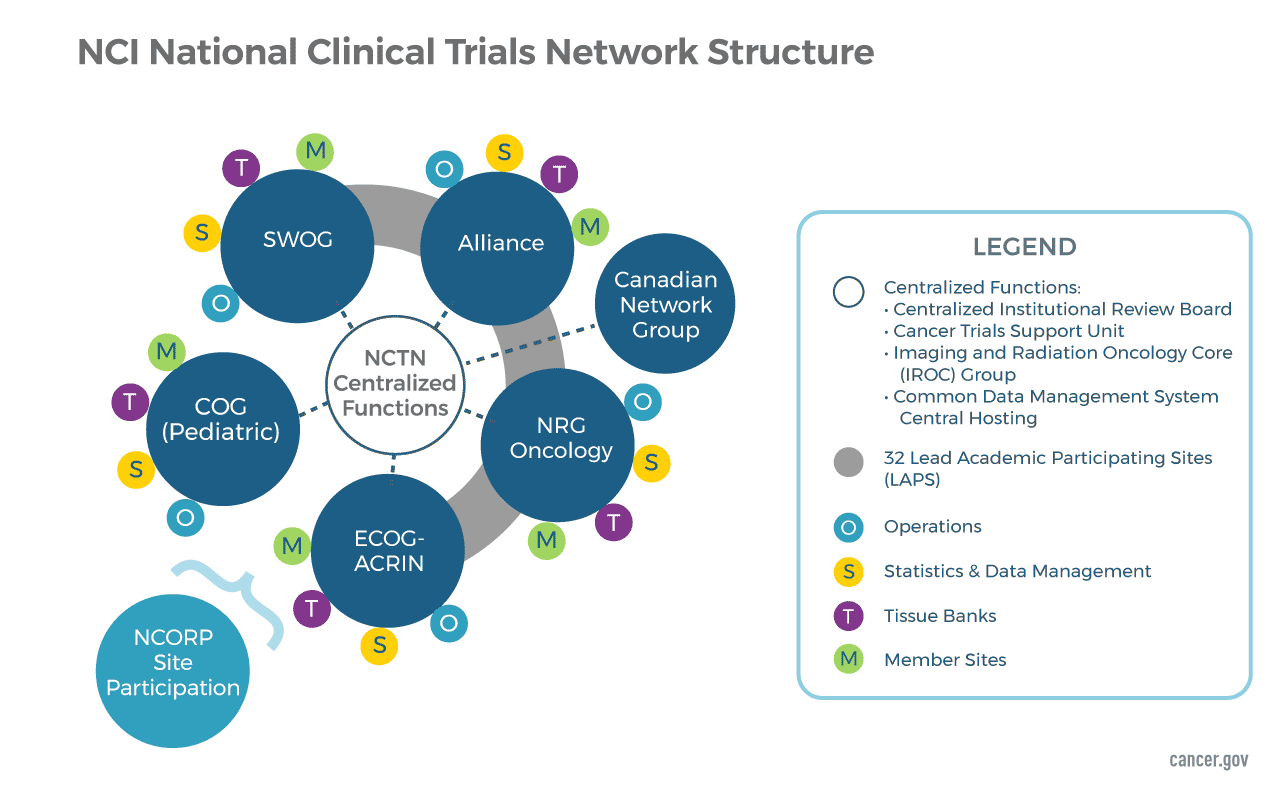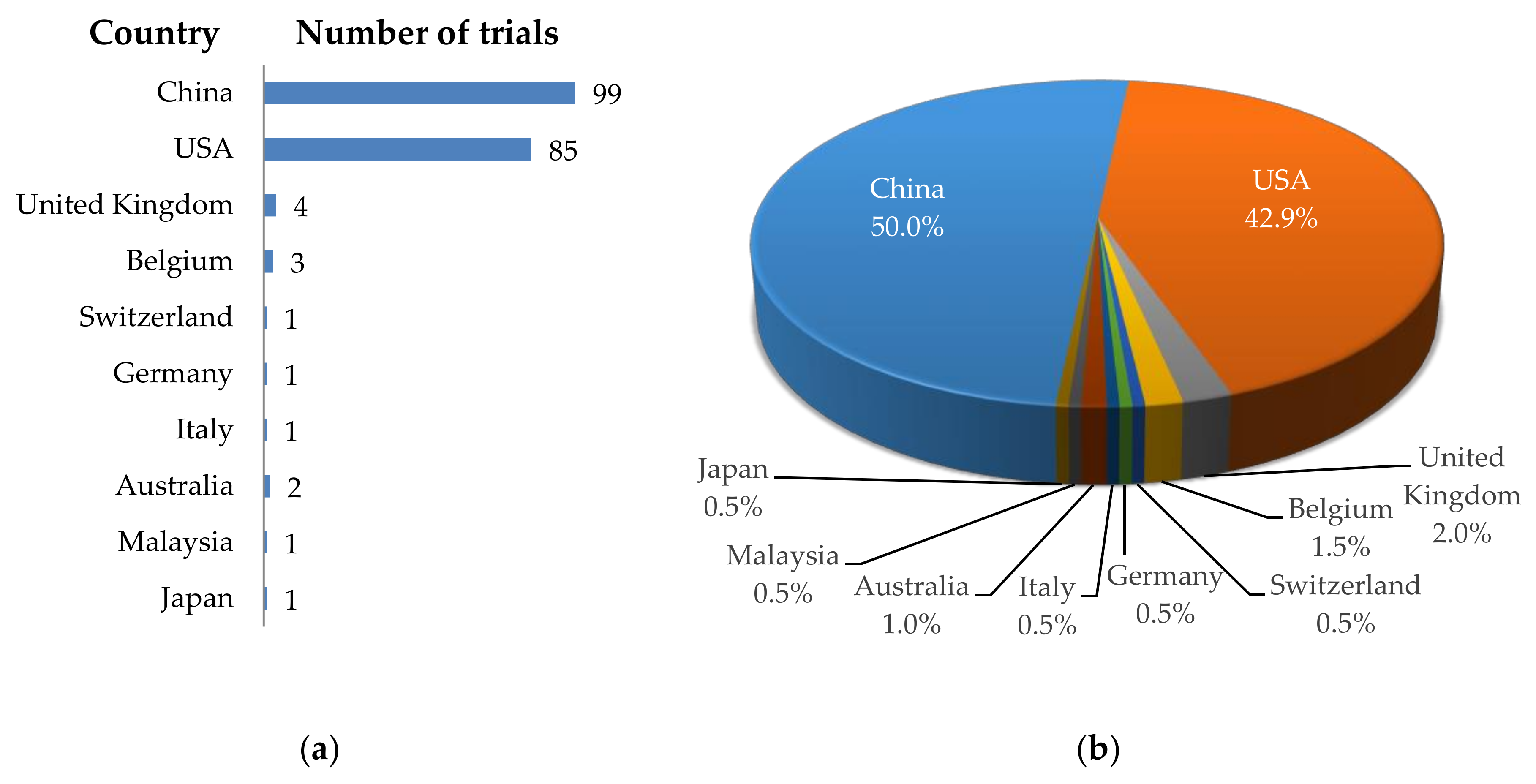
Cancers | Free Full-Text | The Landscape of CAR-T Cell Clinical Trials against Solid Tumors—A Comprehensive Overview | HTML

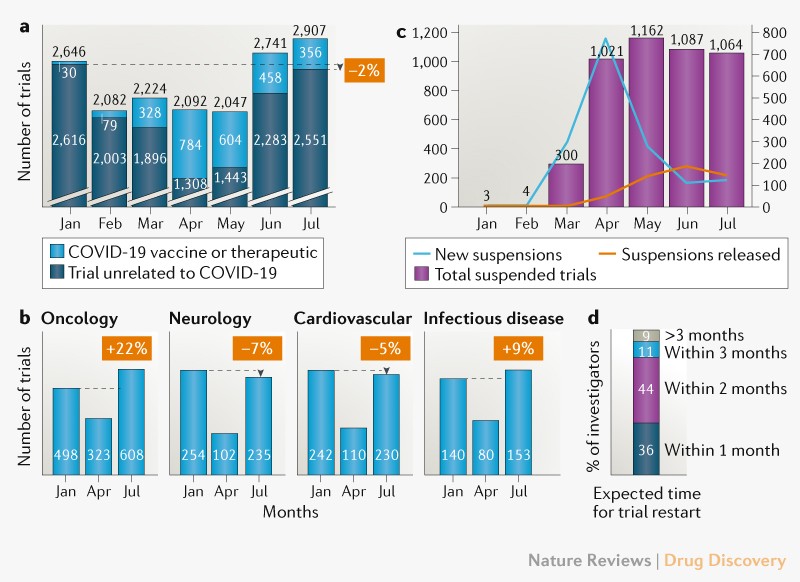
COVID-19's Impact on Oncology Clinical Trials: A One-Year Follow-Up Analysis - Cancer Research Institute (CRI)

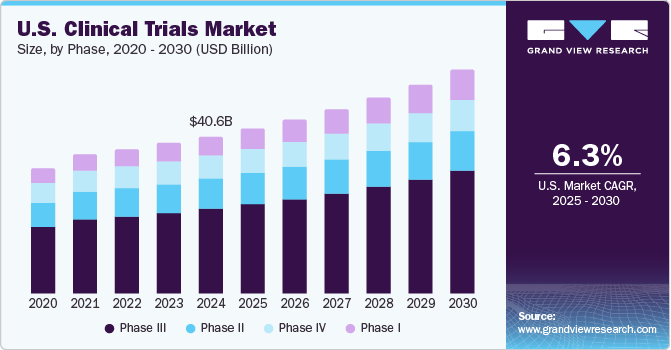
Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine