EMA EudraLex - Volume 4 - GMP Guidelines - TELUGU GMP - Provides GMP Pharmaceutical Guidelines in Telugu.

GMP for medicinal products for human and veterinary use laid down in Commission Directives 91/356/EEC | M A N O X B L O G
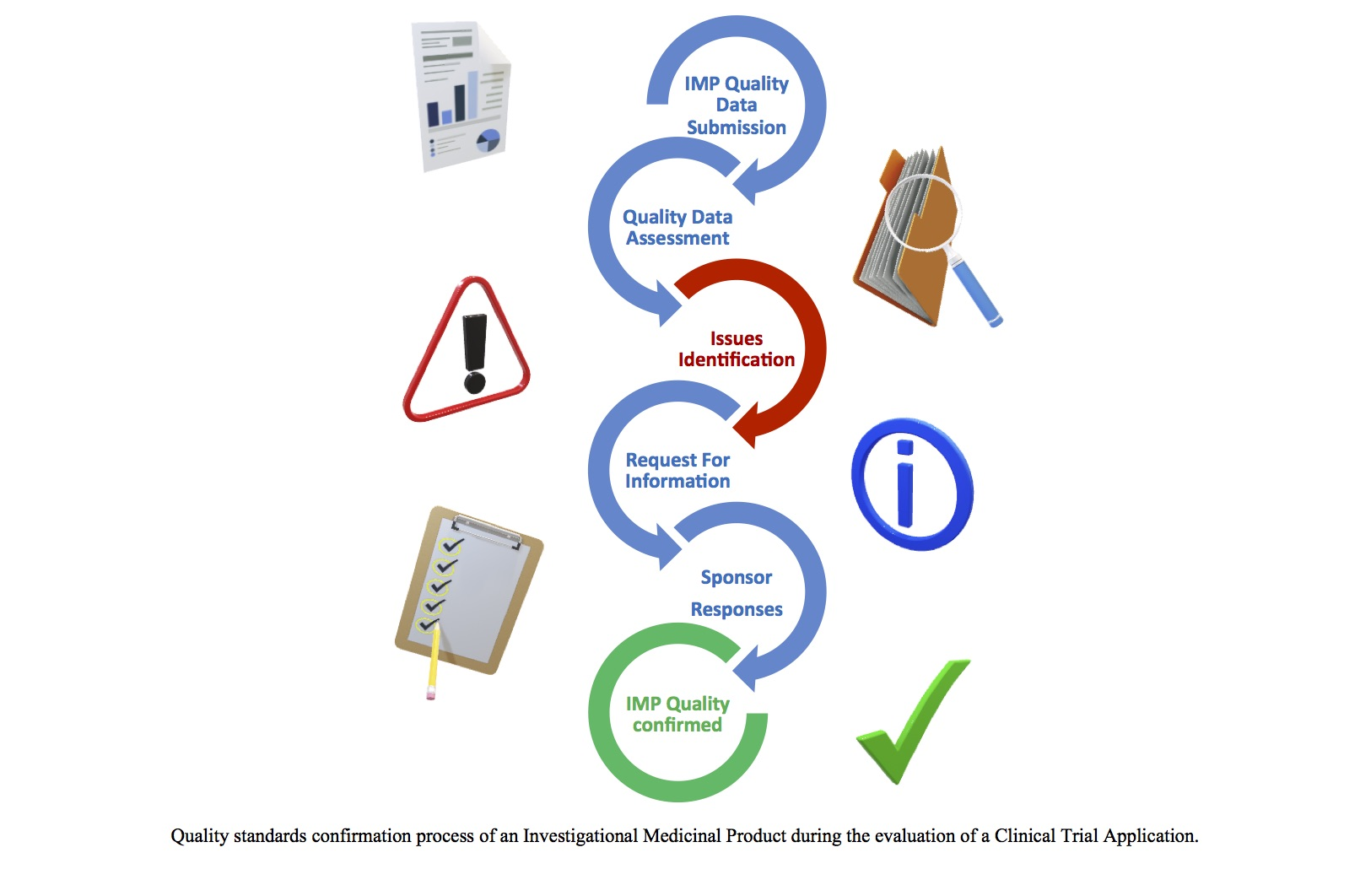
Principal Documents taken into account for the preparation of procedures for GCP inspections requested by the CHMP

Frontiers | Advanced Therapy Medicinal Products' Translation in Europe: A Developers' Perspective | Medicine
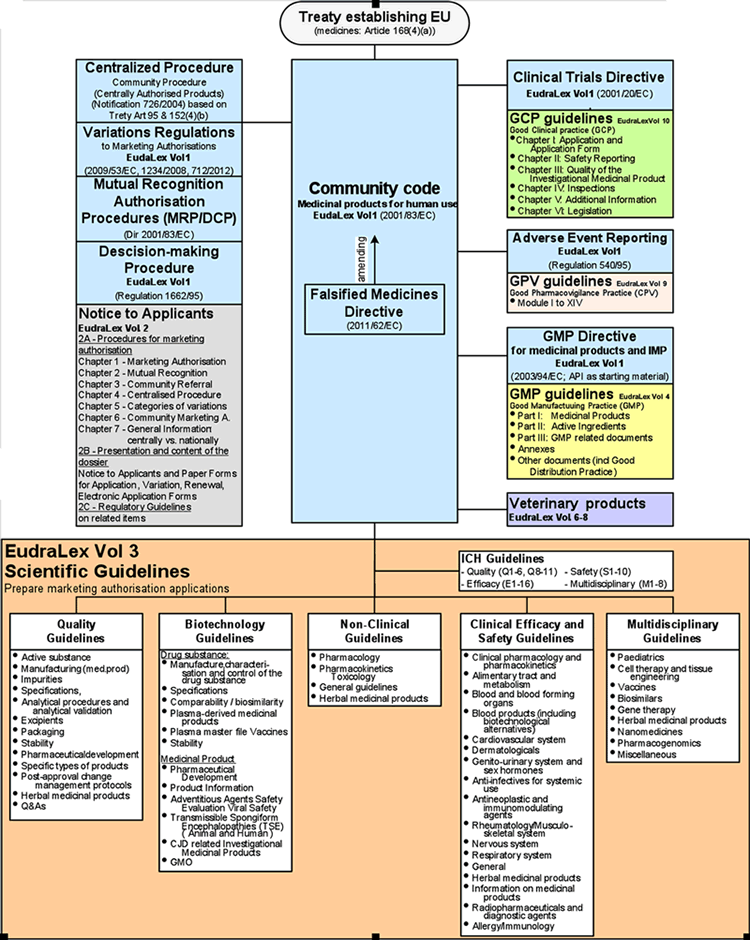
EUROPEAN COMMISSION Brussels, 13 August 2014 Ares(2014)2674284 EudraLex The Rules Governing Medicinal Products in the European U