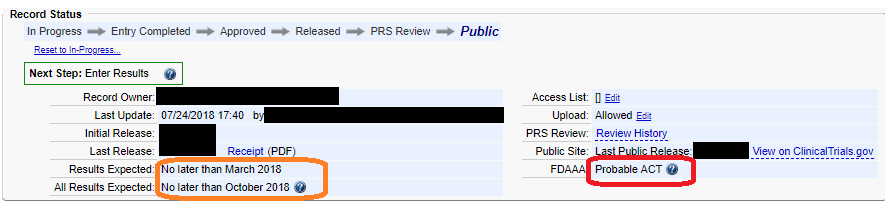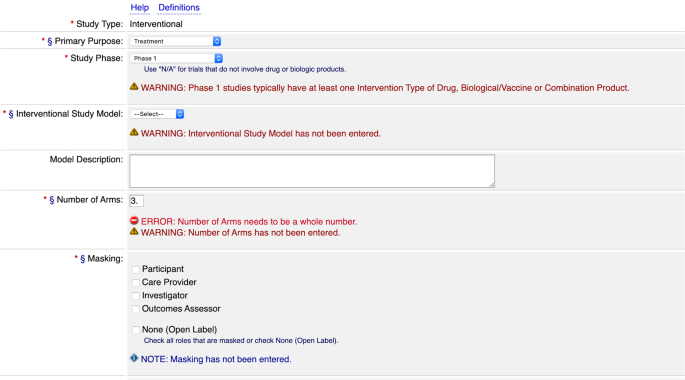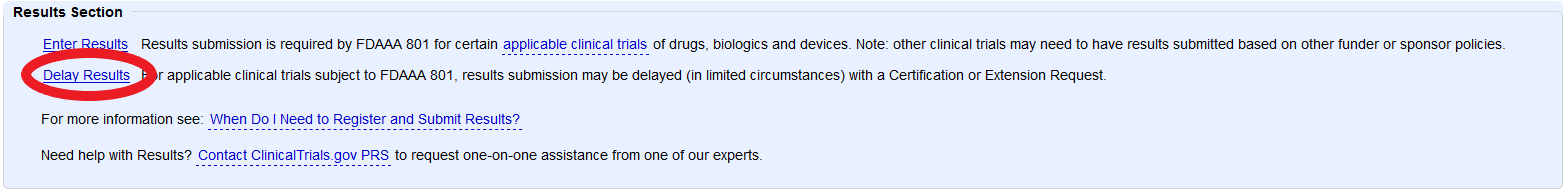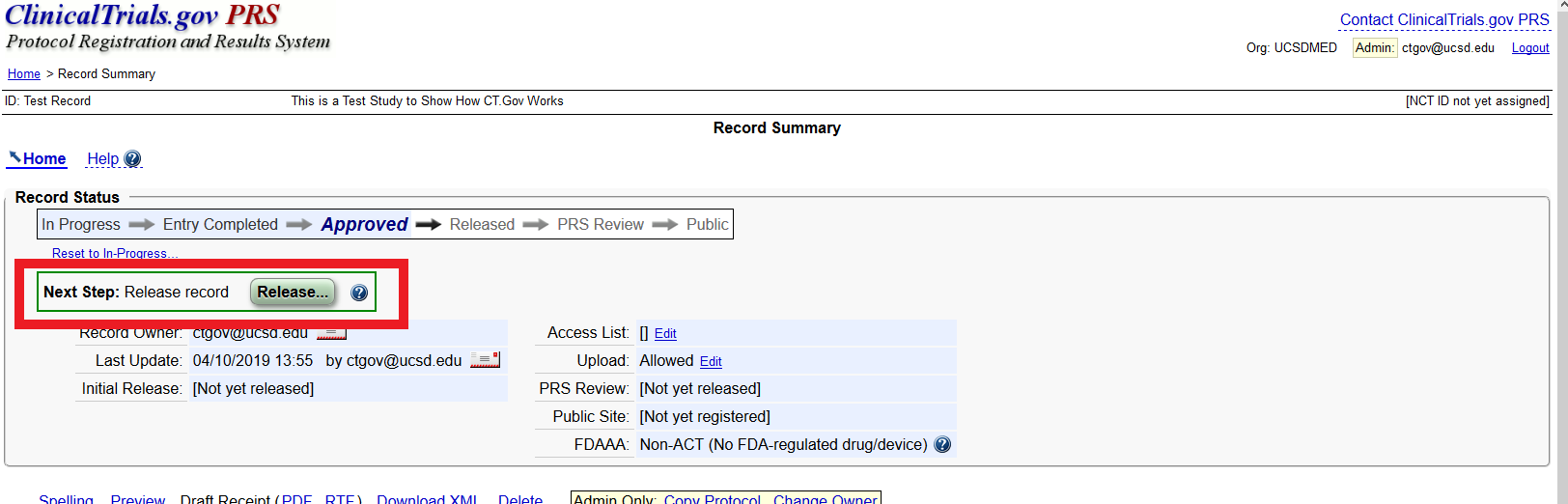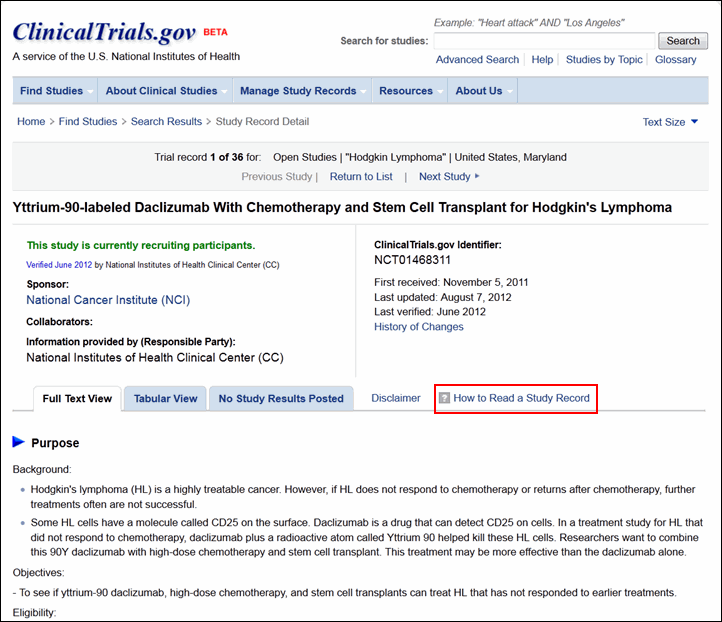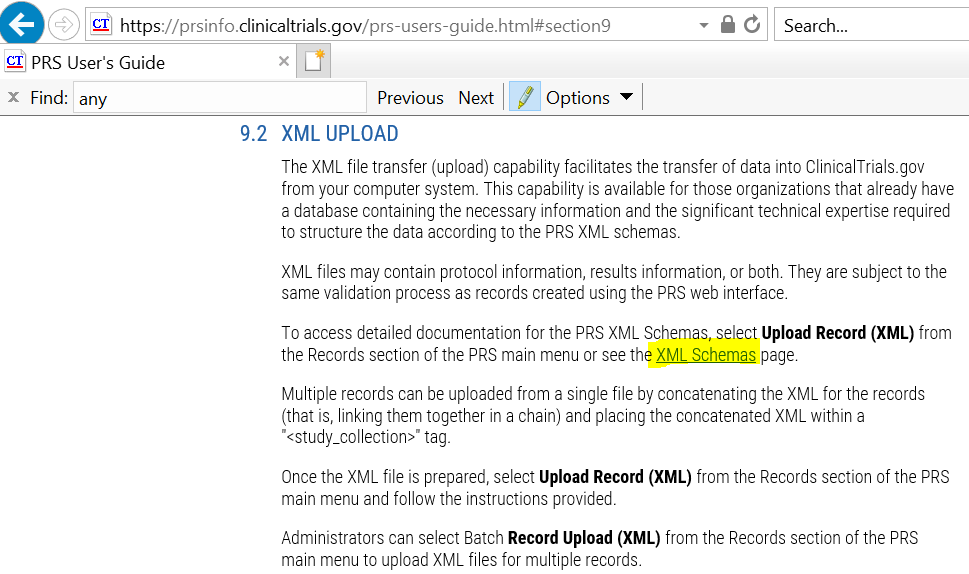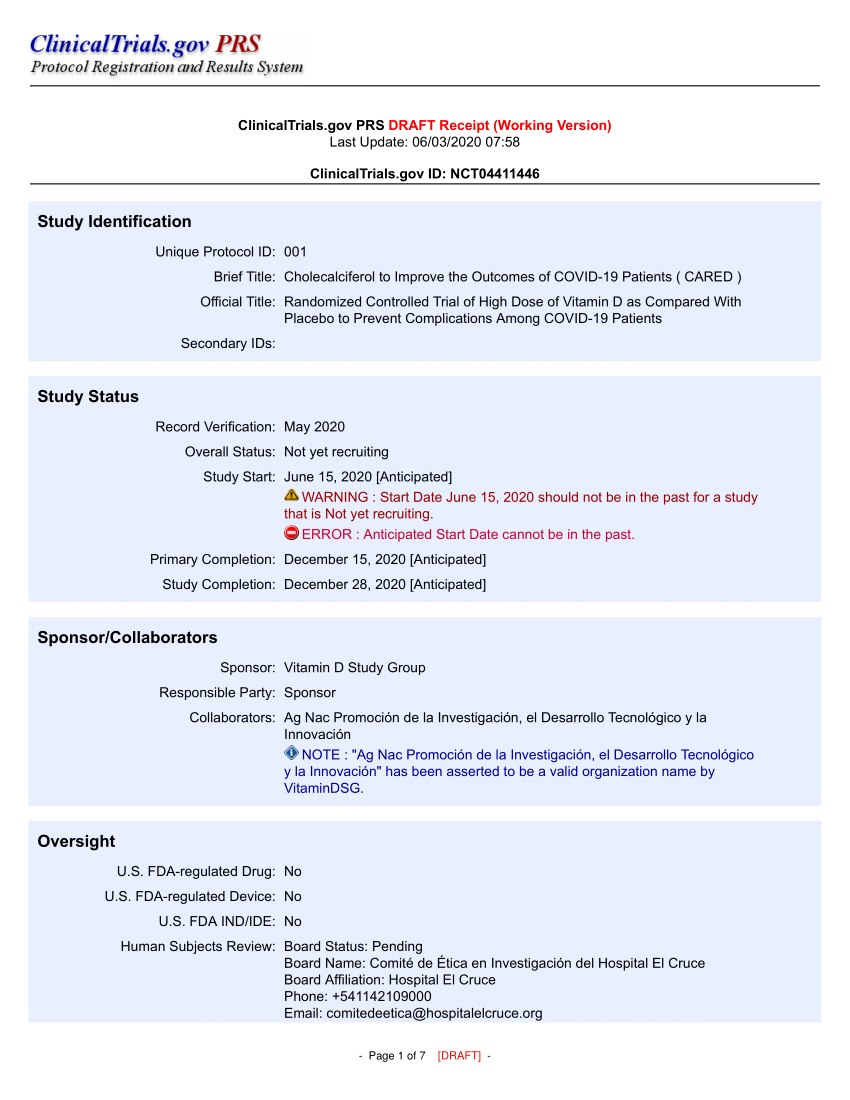
PDF) ClinicalTrials.gov Brief Title: Cholecalciferol to Improve the Outcomes of COVID-19 Patients ( CARED ) Official Title: Randomized Controlled Trial of High Dose of Vitamin D as Compared With Placebo to Prevent

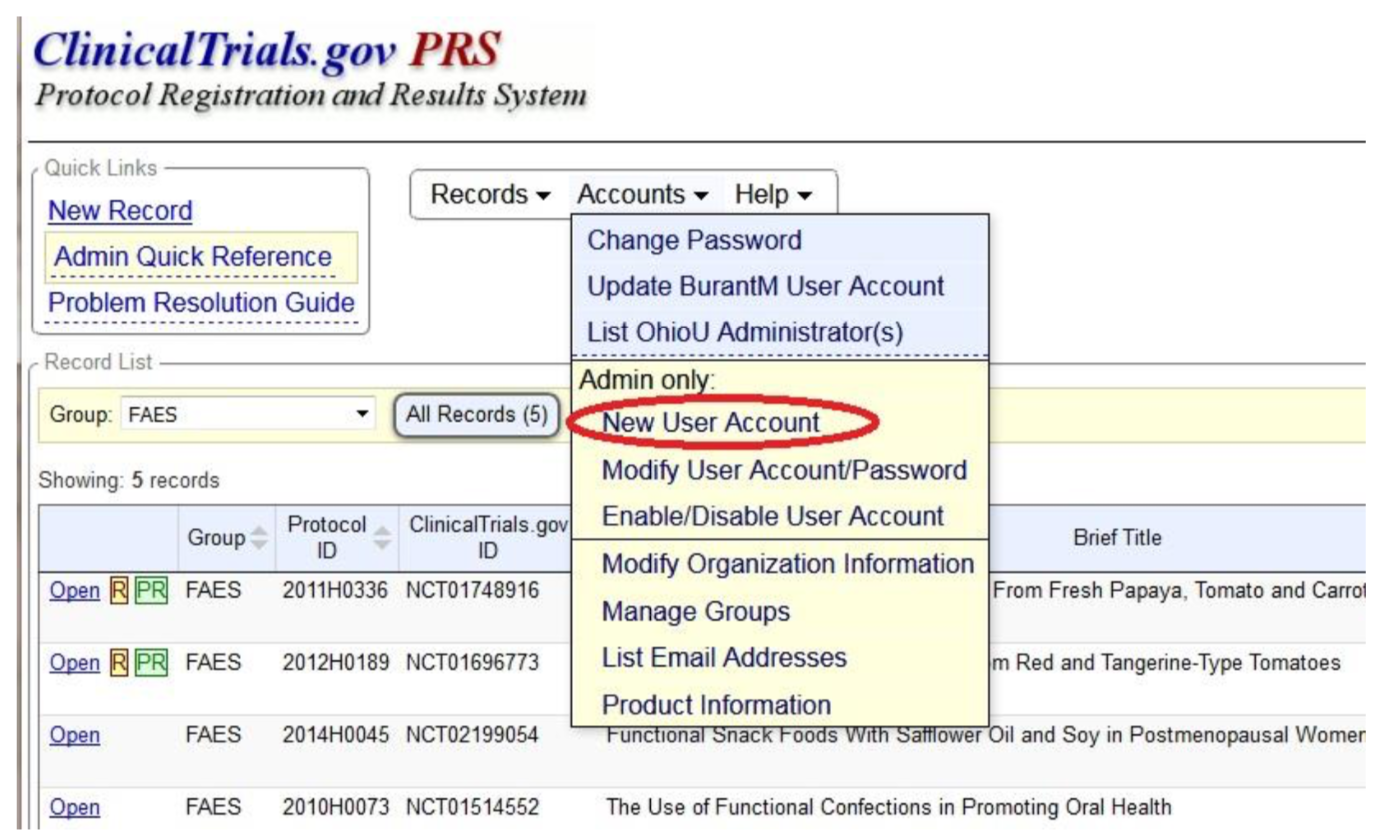
ClinicalTrials.gov: How to Register Your Trial - Clinical and Translational Science Institute - University at Buffalo

ClinicalTrials.gov updates the PRS Guided Tutorials, step-by-step instructions for data providers - NCBI Insights