Comparison between efficacy/safety and pragmatic trials. AE, adverse... | Download Scientific Diagram
Analysis of the reporting of adverse drug reactions in children and adolescents in Germany in the time period from 2000 to 2019 | PLOS ONE
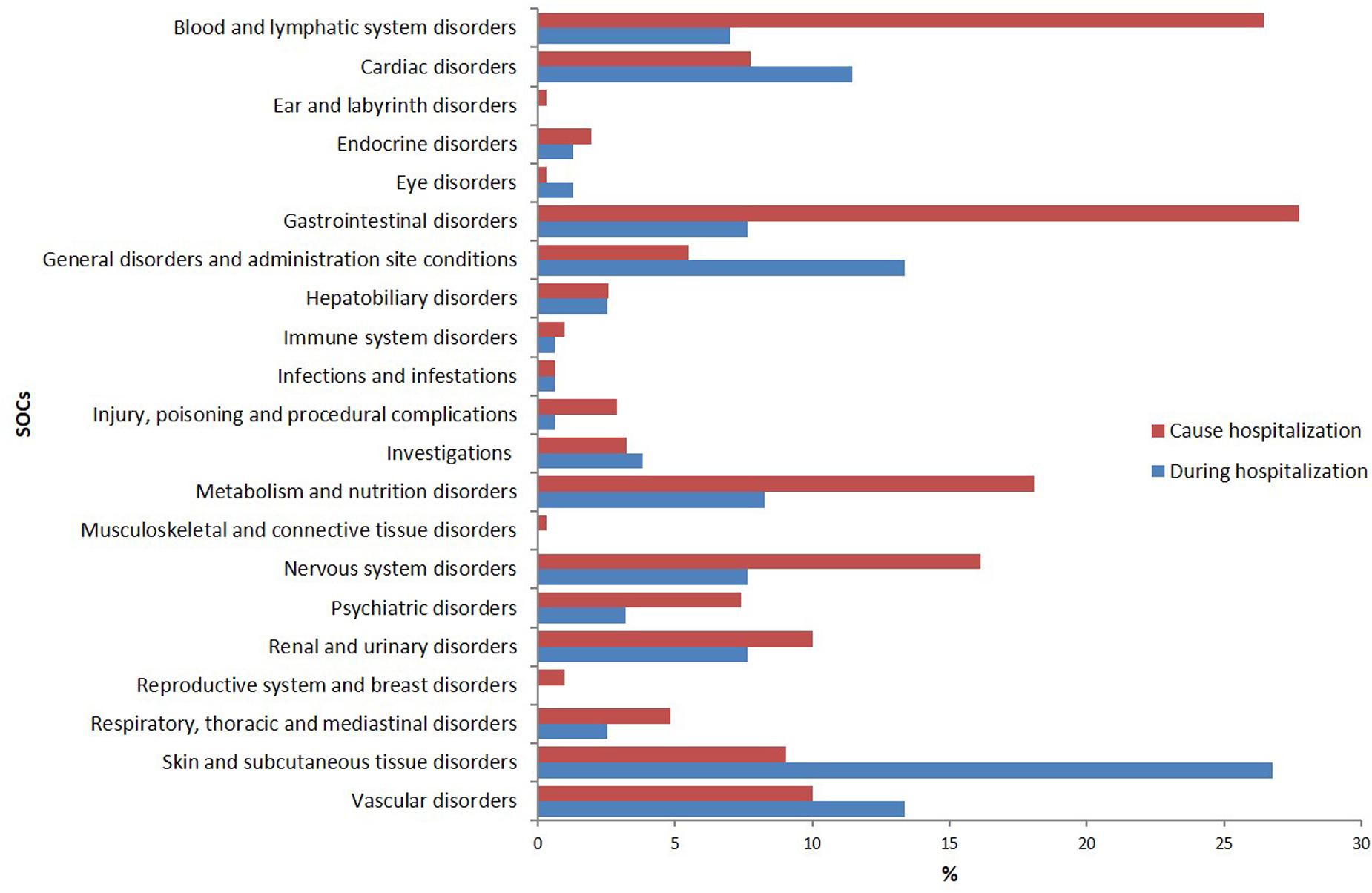
Frontiers | Adverse Drug Reactions in Hospitalized Patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study | Pharmacology
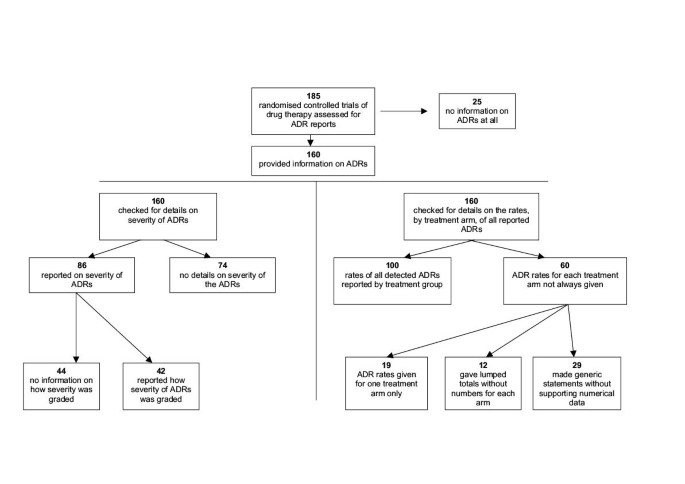
Reporting of adverse drug reactions in randomised controlled trials – a systematic survey | BMC Clinical Pharmacology | Full Text

Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century - eClinicalMedicine

Safety Management Plan – Clinical Trial Medical Monitoring Plan | Online Clinical Research Courses In India

Drug lit ADRs - Drug lit ADR lecture notes - Drug Literature Evaluation Adverse Drug Reaction + Drug - StuDocu

Adverse Drug Reactions to Guideline-Recommended Heart Failure Drugs in Women: A Systematic Review of the Literature - ScienceDirect